Johan Widmark | 2025-05-07 08:00
This commissioned research report is for informational purposes only and is to be considered marketing communication. This research report has not been prepared in accordance with legal requirements designed to promote the independence of investment research and Emergers is not subject to any prohibition on dealing ahead of the dissemination of investment research. This research does not constitute investment advice and is not a solicitation to buy shares. For more information, please refer to disclaimer.
Final dose group confirms tolerability, PK profile
Initial PK data from the highest 30 mg dose in the extended Phase I study of NEX-22 confirm a predictable and dose-dependent exposure, with continued good tolerability and absence of typical GLP-1 side effects such as nausea or vomiting. This supports Nanexa’s aim to offer a once-monthly liraglutide formulation with fewer side effects, a highly attractive proposition for the large type 2 diabetes market. Final patient visits are being completed, with full results expected shortly.
In parallel, Nanexa has ended its long-standing collaboration with Applied Materials, gaining short-term cash while freeing itself from exclusivity in manufacturing equipment. However, Applied Materials’ venture arm, Applied Ventures, still owns some 7 million shares in Nanexa, which it can now be expected to exit, potentially putting non-negligible pressure on the share. Additionally, Bridget Lacey has joined as Chief Business Officer, as the company reports rising interest in the PharmaShell technology and more active partnering discussions.
Valuation and outlook
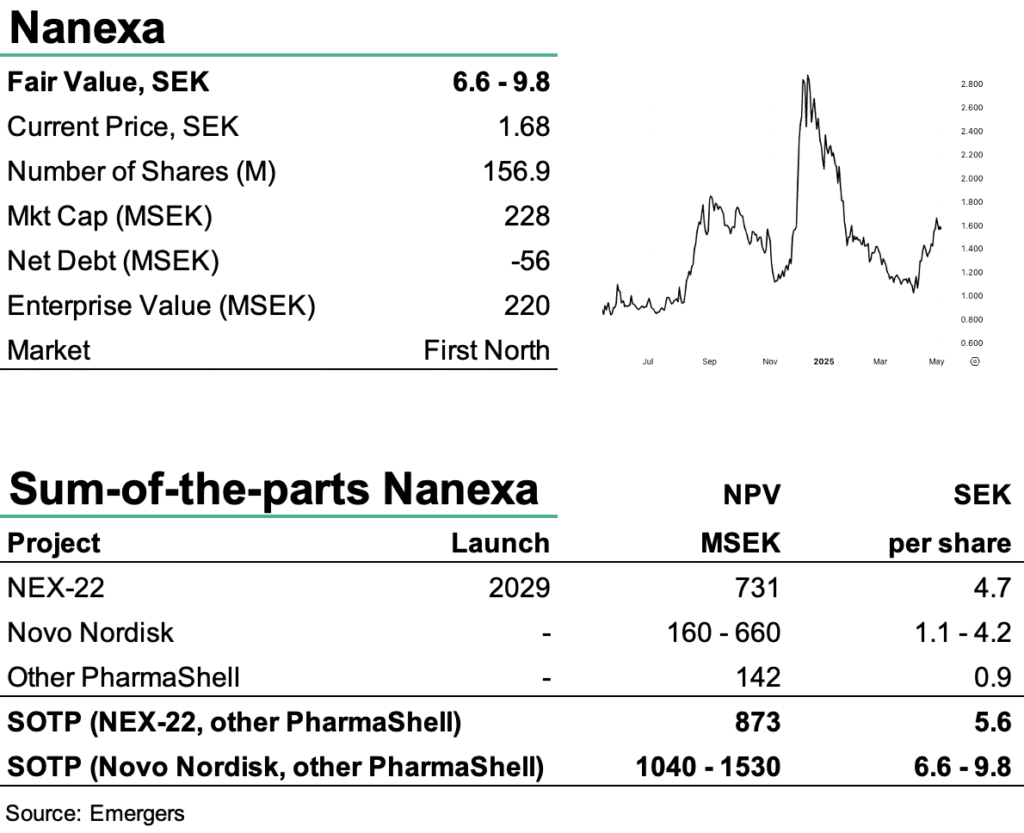
With a strengthened financial position following the raise in Q1’25 and promising Phase I results, we continue to expect treatment of the first patient in the next Phase Ib/II trial before year-end. This trial will be a direct pharmacokinetic comparison of NEX-22 to Victoza, where Nanexa will focus on similarity in order to build on Victoza’s original documentation. If successful, a Pre-IND meeting with the FDA could be held by the end of 2025. After completing Phase III with some 300–400 patients, an application for NEX-22 could realistically be submitted in 2028, with a product on the market by 2029, some three years ahead of any competing long-acting semaglutide drug.
This timeline presents a highly attractive opportunity for potential licensees of NEX-22. Based on this, we continue to find support for an rNPV of SEK 5.6–9.8 per share, driven primarily by NEX-22. However, with few near-term catalysts beyond potential partnering progress, we expect limited share price momentum over the next 6–9 months unless a license deal materialises.
DISCLAIMER