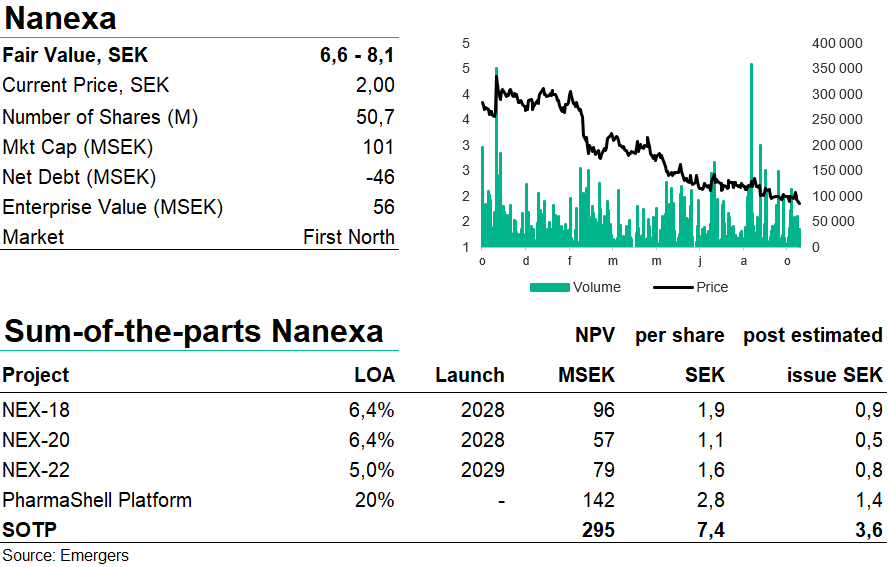
Besides starting its third proprietary development project in Q2 (NEX-22, a long-acting formulation of liraglutide for treatment of type 2 diabetes), Nanexa has also picked up pace in signing new PharmaShell platform evaluation agreements, with two new deals in the last couple of months. With the start of phase I with NEX-20 in Q4’22 (a long-acting injectable of lenalidomide for treatment of multiple myeloma), and phase I with NEX-22 and NEX-18 (long-acting injectable azacitidine for myelodysplastic syndrome) in 2023, we expect an eventful year ahead. We now find support for fair value of SEK 6.6-8.1 per share, before adjustment for a likely capital raise which is drawing closer.

Johan Widmark | 2022-10-26 08:00
This commissioned research report is for informational purposes only and is to be considered marketing communication. This research report has not been prepared in accordance with legal requirements designed to promote the independence of investment research and Emergers is not subject to any prohibition on dealing ahead of the dissemination of investment research. This research does not constitute investment advice and is not a solicitation to buy shares. For more information, please refer to disclaimer.
NEX-22 third proprietary development project
The most noteworthy event during Q3 was the announcement of Nanexa’s third proprietary development project, NEX-22, a long-acting formulation of liraglutide for treatment of type 2 diabetes. Liraglutide is a so-called GLP-1 (Glucagon-like Peptide-1) analogue and today, diabetes treatments are a USD 50 bn market, where GLP-1 analogues account for about USD 15 billion. Today patients on liraglutide take a daily injection of the drug, which Nanexa hopes to replace with a monthly long-acting injectable. One study shows that only 50% of patients suffering from T2D is taking their prescribed injections, which means that improving patient adherence could have significant positive effects on treatment efficacy and cost for the healthcare system. We estimate an rNPV for NEX-22 of around SEK 80m or SEK 1.6 per share. See details in our report New project NEX-22 to add significant daily benefits for 50m patient market.
Two Material Transfer and Feasibility Study Agreements
In Q2, Nanexa also expanded its collaboration agreement with Applied Materials, and received an extended GMP certification for its new production facilities in Uppsala. Importantly, Nanexa has also signed two Material Transfer and Feasibility Study Agreements in the past months, one with a leading global pharma company and another with a specialty pharma company for a depot formulation of a specific compound for intravitreal delivery (into the eye).
Fair value of SEK 6.6-8.1 could be cut in potential issue
With the initiation of phase I with NEX-20 before year end and the restart of clinical trials of NEX-18 and phase I with NEX-22 in 2023, we expect an eventful period ahead. At present, cash position amounts to SEK 45.6m which means that Nanexa is financed for continued development into H1 2023 and that we’re likely to see a capital raise soon. All in all, our estimated risk-adjusted NPV for NEX-18, NEX-20, NEX-22 and the platform PharmaShell (1.9 + 1.1 + 1.6 + 2.8 SEK per share) provide support for a fair value of SEK 6.6-8.1 per share, which a potential issue at SEK 1.50 per share could cut to SEK 3.6, still providing a significant upside with plenty of milestones ahead.
DISCLAIMER