Magnus Brolin & Johan Widmark | 2022-06-03 08:00
This commissioned research report is for informational purposes only and is to be considered marketing communication. This research report has not been prepared in accordance with legal requirements designed to promote the independence of investment research and Emergers is not subject to any prohibition on dealing ahead of the dissemination of investment research. This research does not constitute investment advice and is not a solicitation to buy shares. For more information, please refer to disclaimer.
2.5 MEUR in funding for ELC-301
Elicera is a clinical phase cell and gene therapy company developing immune-oncology therapies focusing on amplified CART T-cells and oncolytic viruses. Elicera now estimate the planned clinical phase I/II for the development of CAR T-cell therapy ELC-301 to be fully financed, after being granted 26 MSEK from the European Innovation Council (EIC) Accelerator Programme. With the grant, the company has now secured 40 MSEK in total for the ELC-301-program which we expect to enter clinical phase I/II in H2’22. The EIC-accelerator program offers funding support and acceleration services for small and mid-sized companies with an approval rate of less than five percent, which is why we believe that the grant offers considerable validation to Elicera’s science and research.
Elicera armed with a broad product portfolio
The approved CAR T-cell therapies available today target the CD19-protein and while those have proven to be effective, a large portion of patients are either resistant to the treatment or relapse within twelve months. These patients have then often lost the CD19 target antigen on the recurring tumors. ELC-301, which is the fourth generation CAR T-cell therapy directs toward the CD20-protein instead, which is expressed on all B-cell lymphoma cells. ELC-301 is also armed with Elicera’s own CAR T-cell amplifying platform technology iTANK which offers a broader attack on cancer by also activating the patient’s own T-cells against the whole set of relevant target antigens on tumor cells, not just CD19 or CD20. In addition to ELC-301 and ELC-100 Elicera have two additional drug candidates in its portfolio ELC-201 and ELC-401, both in the pre-clinical phase.
Stable pipeline of triggers support revaluation potential
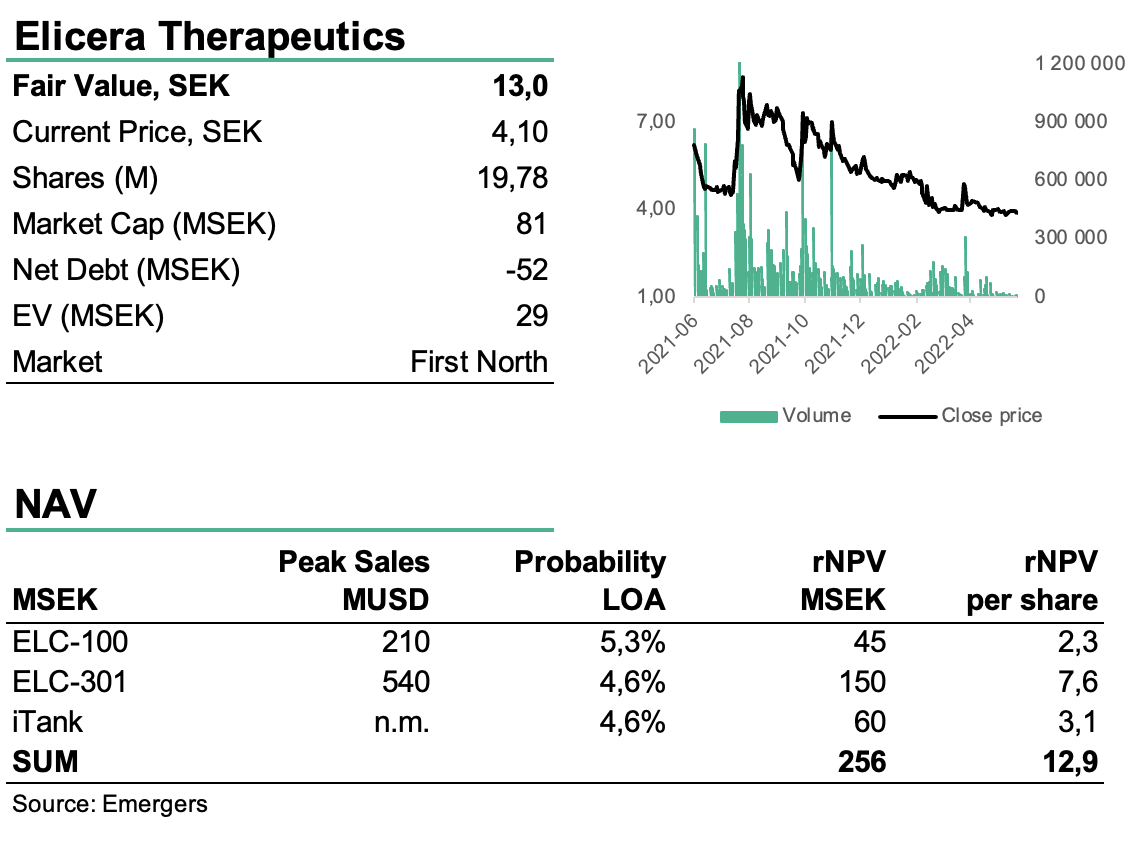
For Elicera’s other project, ELC-100, an oncolytic virus for treatment of neuroendocrine tumors, we now look forward to efficacy data from the ongoing phase I/II study during 2022. With a portfolio of immune-oncology projects in clinical and pre-clinical phases, as well as the iTANK platform which is already in the commercial phase, and a stable pipeline of triggers during the year, plus 1.3 SEK per share in EIC-grants for the forthcoming phase I/II with ELC-301, we find ample support for a revalaution of the share. The company’s strategy is focused on partnerships after initial clinical phases and with effective measures and potential for early out-licensing for ELC-100, the start of clinical studies for ELC-301 and probable pilot agreements for iTANK in 2022, we see an overall risk-adjusted net present value for these three verticals at 256 MSEK or a fair value 13 (12) SEK per share.
DISCLAIMER